- Theranostic Insights🧪
- Posts
- Theranostic Newsletter | 12.2025 Edition
Theranostic Newsletter | 12.2025 Edition
Information & Insights From The Experts
Here’s your latest dose of Theranostic Insights—a curated snapshot of what’s new and next in the world of theranostics powered by TheranosticTrials.org. From clinical breakthroughs to tech trends, we’ve got the highlights you need to stay ahead.
Latest Theranostic News 📰
Bayer Acquires Pan-Amyloid Imaging Agents to Expand Molecular Diagnostics in Cardiac Disease
Bayer has acquired two investigational tracers from Attralus—AT-01, a PET agent in Phase III, and AT-05, a SPECT agent in Phase I—to enhance early diagnosis of cardiac and systemic amyloidosis. AT-01 is the first pan-amyloid imaging agent to receive FDA Breakthrough Therapy Designation for cardiac amyloidosis. This strategic move strengthens Bayer’s position in molecular imaging and precision cardiology, advancing theranostic capabilities in underdiagnosed cardiovascular conditions. Continue reading here
Curium Expands Theranostic Reach with New Legal Entity in China
Curium has launched Curium Shanghai Pharmaceuticals Co., Ltd., marking a significant step in expanding access to theranostic radiopharmaceuticals across Asia. This move strengthens Curium’s ability to collaborate with local partners and enhance the delivery of cancer diagnostics and therapies in the Chinese market. The company’s local presence will support the development of next-generation theranostic agents targeting a wide range of modalities and isotopes. Continue reading here
Ratio Therapeutics Begins Clinical Trial of FAP-Targeted Alpha Therapy for Sarcoma
Ratio Therapeutics has dosed the first patient cohort in its Phase 1/2 ATLAS trial evaluating [Ac-225]-RTX-2358, a targeted alpha radiopharmaceutical directed at fibroblast activation protein (FAP) in relapsed or refractory soft tissue sarcomas. The therapy uses actinium-225 to deliver high-precision radiation to FAP-expressing tumors, with initial imaging confirmation via [Cu-64]-labeled PET scans. This marks a key step in expanding theranostic strategies to rare and aggressive cancers with few existing treatment options. Continue reading here
AdvanCell Receives First Delivery of High-Activity Thorium-228, Highlighting a Unique Industry Capability and Expertise Essential to Scaling Lead-212 Production for Targeted Alpha Therapies
A major manufacturing milestone for AdvanCell with the first delivery of high-activity thorium-228, the critical parent isotope for producing lead-212 used in targeted alpha therapies. This capability supports large-scale, reliable production of 212Pb for ADVC001 and other pipeline programs, strengthening AdvanCell’s vertically integrated theranostic and theranostics development platform. The advance positions the company to meet growing clinical demand and accelerate late-stage trials of lead-212–based radioligand therapies. Continue reading here
Orano Med and Roche Advance Two-Step Alpha Radioimmunotherapy for CEA-Positive Cancers
Orano Med and Roche are entering clinical development for a next-generation two-step pretargeted radioimmunotherapy (PRIT) using lead-212 (²¹²Pb) to treat carcinoembryonic antigen (CEA)-expressing cancers, including colorectal, pancreatic, and gastric tumors. The approach separates antibody targeting and radioligand delivery to improve precision and reduce radiation to healthy tissue. Preclinical data show strong tumor uptake and reduced off-target toxicity, highlighting the potential of this theranostic strategy to improve outcomes in hard-to-treat cancers. Continue reading here
ARTBIO Doses First Patients in Phase 1 Trial of 212Pb Alpha Therapy for Advanced Prostate Cancer
ARTBIO has initiated dosing in two patient cohorts of its ARTISAN Phase 1 trial evaluating AB001, a PSMA-targeted alpha radioligand therapy labeled with lead-212, for metastatic castration-resistant prostate cancer (mCRPC). The trial includes patients both naïve to and previously treated with ¹⁷⁷Lu-PSMA therapy. AB001 represents a novel theranostic approach aiming to expand treatment options for mCRPC patients with limited alternatives. Continue reading here
Radioligand therapy of pancreatic ductal adenocarcinoma using an αvβ6-integrin targeting 68Ga / 177Lu labeled theranostic pair
A first-in-human experience using an αvβ6-integrin–targeted theranostic approach in metastatic pancreatic ductal adenocarcinoma, a cancer with few effective treatment options. PET imaging with 68Ga confirmed strong tumor uptake, while subsequent therapy with 177Lu-Therahexin-503 showed high and prolonged retention in metastatic lesions, favorable biodistribution, and good tolerability without relevant side effects. These findings suggest that αvβ6-integrin–targeted theranostics may offer a promising new radioligand therapy strategy for pancreatic cancer and other αvβ6-expressing tumors. Continue reading here
AdvanCell Launches Phase 2 Trial of Lead-212 Alpha Therapy for Prostate Cancer
AdvanCell has initiated the Phase 2 expansion of its TheraPb trial to evaluate ADVC001, a novel PSMA-targeting alpha radioligand therapy labeled with lead-212 (²¹²Pb), in patients with metastatic prostate cancer. Early data showed strong safety and anti-tumor activity, prompting exploration of two therapeutic dose levels across three settings, including treatment after ¹⁷⁷Lu-PSMA therapy. This next phase strengthens the role of targeted alpha therapies in theranostic strategies for prostate cancer. Continue reading here
ATNM-400 Targets B7-H3 and Is Being Studied in a Phase 1 Clinical Trial in South Africa
Actinium Pharmaceuticals is moving into solid tumors with ATNM-400, a B7-H3 targeted radiotherapy now in a Phase 1 trial in South Africa to assess safety, where the drug goes in the body, and early anti-tumor signals in advanced B7-H3 positive cancers. Because B7-H3 is often overexpressed in breast cancer and non-small cell lung cancer and linked to aggressiveness and immune escape, this theranostic approach could offer a precision option when standard treatments fail. If early data are positive, ATNM-400 may anchor a broader theranostics pipeline across multiple solid tumors. Continue reading here
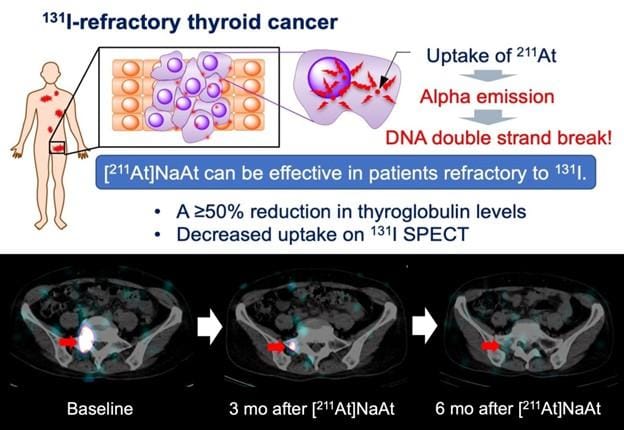
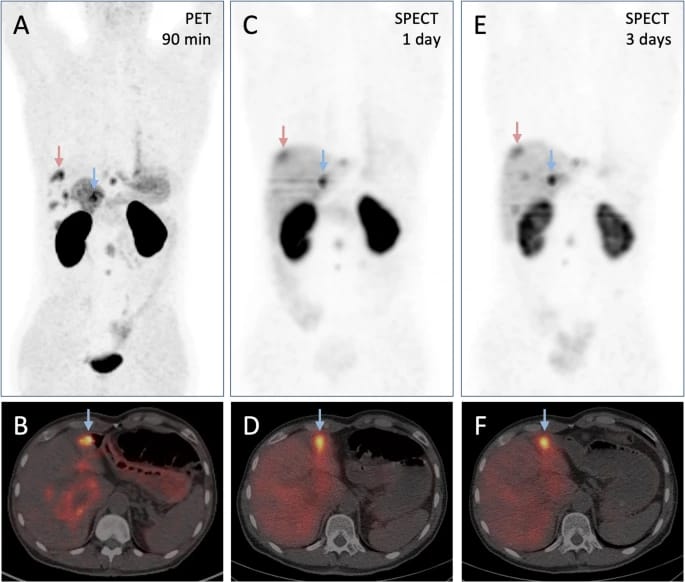
Astatine-Based Alpha Therapy Offers New Hope for Radioiodine-Resistant Thyroid Cancer
A first-in-human trial has shown that 211At-NaAt, a targeted alpha therapy using astatine-211, is safe and effective in patients with differentiated thyroid cancer that has become refractory to radioactive iodine. A single intravenous dose achieved disease control in some patients without the need for daily molecular-targeted drugs, offering a better-tolerated alternative to standard treatments. This advancement highlights the growing potential of alpha-emitting radiopharmaceuticals in theranostic applications. Continue reading here
Global PET Accreditation Framework Launched to Standardize Theranostic Imaging
SNMMI, EANM, and ARTnet have introduced a unified PET/CT and PET/MR accreditation system that will standardize quantitative imaging across clinical trials and healthcare worldwide. The new contrast recovery coefficient (CRC)-based framework replaces older methods, aiming to improve data quality, reduce duplication, and accelerate site accreditation. This harmonized approach will strengthen the reliability of PET imaging for theranostic development and patient care. Continue reading here
PharmaLogic Delivers First Clinical Supply of FAP-Targeted Alpha Therapy for Sarcoma Trial
PharmaLogic has successfully manufactured and delivered the first clinical doses of [Ac-225]-RTX-2358, a FAP-targeted radiopharmaceutical developed by Ratio Therapeutics, for the Phase 1/2 ATLAS trial in aggressive soft tissue sarcomas. The doses were produced at PharmaLogic’s Bronx facility, supporting the rapid clinical advancement of actinium-225–based theranostic therapies. This milestone highlights the critical role of radiopharmaceutical manufacturing in expanding access to precision cancer treatments.
Continue reading here
Cellectar Secures Actinium-225 and Astatine-211 Supply to Advance Alpha Therapies for Solid Tumors
Cellectar Biosciences has signed a multi-year agreement with Ionetix to ensure clinical and commercial supply of actinium-225 and astatine-211 for its targeted alpha therapy programs. The agreement supports the development of CLR-225, a phospholipid radioconjugate designed to treat solid tumors such as pancreatic cancer with precise tumor-targeted radiation. Reliable isotope access will accelerate Cellectar’s theranostic pipeline and expand the use of alpha-emitting agents in oncology. Continue reading here
First-in-Human PET Imaging with 64Cu-Macrin Highlights Theranostic Potential in Macrophage-Rich Diseases
A first-in-human trial of the nanoparticle imaging agent ⁶⁴Cu-Macrin demonstrated safety and effective targeting of macrophage-rich tissues in both healthy individuals and patients with cancer or sarcoidosis. The agent showed favorable biodistribution and pharmacokinetics, with notable uptake at active disease sites, supporting its potential for theranostic use in immune-related conditions. This macrophage-specific imaging approach could aid in patient selection and monitoring for macrophage-targeted therapies. Continue reading here
AtomVie and Ionetix Partner to Expand Actinium-225 Supply for Theranostic Development
AtomVie Global Radiopharma has signed a supply agreement with Ionetix to secure cGMP-grade actinium-225 for its growing portfolio of targeted alpha radiopharmaceuticals. The partnership supports AtomVie's upcoming manufacturing facility in Canada and enhances global access to Ac-225, a key isotope in advancing theranostic therapies for cancer. This integrated supply chain strengthens the infrastructure needed for scalable alpha-emitting treatments. Continue reading here
BAMF Health and Henry Ford Health to Open Theranostics Center in Detroit
BAMF Health and Henry Ford Health have partnered to launch a 45,000-square-foot Comprehensive Theranostics Center in downtown Detroit, focused on delivering advanced molecular imaging, targeted radiopharmaceutical therapy, and clinical research. The new facility will expand patient access to precision medicine and bolster Michigan’s position as a leader in theranostics. By integrating care, research, and manufacturing, the center aims to make cutting-edge therapies more accessible and affordable. Continue reading here
Terbium-161 Shows Superior Tumor Dose in Neuroendocrine Tumors Compared to Lutetium-177
A Phase 0 trial comparing [¹⁶¹Tb]Tb-DOTA-LM3 to [¹⁷⁷Lu]Lu-DOTATOC in patients with somatostatin receptor–positive gastroenteropancreatic neuroendocrine tumors found that terbium-161 delivered over seven times more radiation to tumors while maintaining a comparable safety profile. The enhanced tumor uptake and added emission of short-range Auger and conversion electrons position ¹⁶¹Tb as a potent theranostic radionuclide, particularly effective against micrometastases and heterogeneous tumors. Continue reading here
RadioMedix Secures Long-Term Thorium-228 Supply to Advance Alpha Therapy Pipeline
RadioMedix has signed a five-year agreement with Van Overeem Nuclear for thorium-228, a key precursor for producing lead-212 used in targeted alpha therapies. The supply will fuel production via RadioMedix’s proprietary RAHA-100 generator and support development of novel radiopharmaceuticals. The company's newly opened SPICA Center in Houston will further enable integrated theranostic manufacturing and research.
Continue reading here