- Theranostic Insights🧪
- Posts
- Theranostic Newsletter | 09.2025 Edition
Theranostic Newsletter | 09.2025 Edition
Information & Insights From The Experts
Here’s your latest dose of Theranostic Insights—a curated snapshot of what’s new and next in the world of theranostics powered by TheranosticTrials.org. From clinical breakthroughs to tech trends, we’ve got the highlights you need to stay ahead.
Latest Theranostic News 📰
Clarity Confirms U.S.-Based Manufacturing in Response to New Tariff on Pharmaceuticals
Clarity Pharmaceuticals has confirmed that its Targeted Copper Theranostic (TCT) products and clinical trials in the U.S. will not be impacted by the newly announced 100% tariff on imported branded pharmaceuticals. With five active INDs and all isotope and drug product manufacturing located in the U.S., Clarity has built a fully domestic supply chain to support its Phase III diagnostic and Phase I/IIa theranostic trials. This strategy ensures resilience against political trade dynamics while advancing next-generation theranostic treatments for cancer patients. Continue reading here
TerraPower, Evergy, and Kansas Announce Agreement to Explore Advanced Nuclear Energy Deployment
TerraPower, Evergy, and the Kansas Department of Commerce have signed an MOU to evaluate deploying TerraPower’s Natrium® advanced nuclear reactor and energy storage system in Kansas. The Natrium design combines a sodium-cooled fast reactor with molten salt energy storage, enabling flexible, carbon-free power generation with grid reliability. This collaboration underscores Kansas’ commitment to an all-of-the-above energy strategy and could position the state as a leader in next-generation nuclear innovation. Continue reading here
Orano Med Achieves Key Milestone in French ATEF Facility Construction for Large-Scale Lead-212 Production
Orano Med has completed the watertight phase of its Advanced Thorium Extraction Facility (ATEF) in France, a major step toward large-scale production of thorium-228, the precursor to lead-212 used in targeted alpha therapies. Scheduled to begin operations in 2027, ATEF will be the world’s first industrial facility dedicated to this critical isotope, boosting production capacity tenfold compared to current levels. Once fully operational, the site could help supply innovative theranostic treatments to up to 25,000 cancer patients annually. Continue reading here
Telix and FDA Agree on Resubmission Pathway for TLX101-CDx (Pixclara®) U.S. NDA
Telix has reached an agreement with the FDA on the resubmission of its New Drug Application for TLX101-CDx (Pixclara®), an investigational PET imaging agent for glioma. The resubmission package will include an additional confirmatory efficacy study analysis to address FDA concerns outlined in a prior Complete Response Letter. With expedited review likely, TLX101-CDx could soon provide a critical diagnostic tool to improve management of glioma, advancing the role of theranostics in brain cancer care. Continue reading here
Lantheus and GE HealthCare Announce Exclusive Licensing Agreement for Prostate Cancer Imaging Agent PYLARIFY® in Japan
Lantheus has granted GE HealthCare exclusive rights to develop, manufacture, and commercialize its PSMA-targeted PET imaging agent PYLARIFY® (piflufolastat F18) in Japan. Already the leading PSMA PET tracer in the U.S. and Europe, PYLARIFY helps identify prostate-specific membrane antigen–positive lesions in men with prostate cancer, guiding diagnosis and treatment decisions. By leveraging GE HealthCare’s manufacturing capabilities and regional expertise, this partnership expands access to a proven theranostic tool in one of the world’s largest prostate cancer markets. Continue reading here
Perspective Therapeutics Progresses Dose Finding for [212Pb]VMT01 in Combination with Nivolumab in Metastatic Melanoma
Perspective Therapeutics has advanced to a higher-dose cohort in its Phase 1/2a trial of [212Pb]VMT01, a targeted alpha therapy, in combination with the PD-1 inhibitor nivolumab for MC1R-positive metastatic melanoma. The decision follows early safety and anti-tumor activity observed at lower doses, prompting evaluation of 3.0 mCi dosing both as monotherapy and in combination. This study highlights the potential synergy between alpha-emitting theranostics and immunotherapy, offering hope for patients with few treatment options. Continue reading here
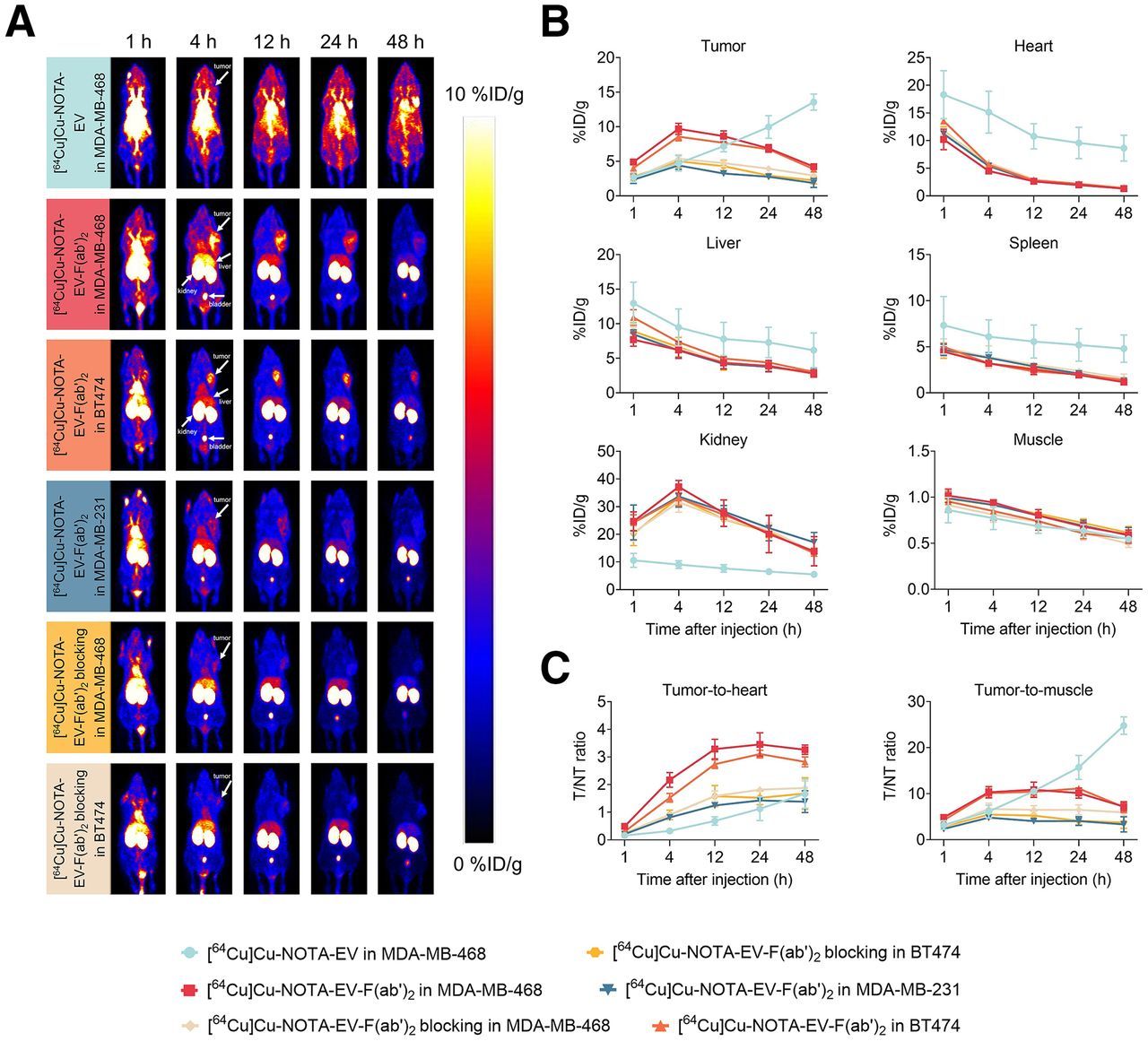
New PET Tracer Enables Same-Day Imaging of Triple-Negative Breast and Urothelial Cancers
Researchers have developed a PET tracer that can visualize nectin-4 expression in triple-negative breast and urothelial bladder cancers within just four hours, enabling same-day imaging. In preclinical models, the tracer 64Cu-NOTA-EV-F(ab′)2 showed rapid tumor uptake, high specificity, and superior tumor-to-background ratios compared with full-length antibody tracers. This innovation could accelerate patient stratification for nectin-4–targeted therapies and improve real-time monitoring, advancing the role of theranostics in aggressive cancers. Continue reading here
Lesion Analysis of 18F-Metafluorobenzylguanidine PET Imaging in Neuroblastoma
A new study shows that 18F-MFBG PET imaging detects more neuroblastoma lesions than the standard 123I-MIBG scan, with faster same-day results. In 37 patients, 18F-MFBG identified additional disease sites in most cases and confirmed more true-positive lesions, demonstrating higher sensitivity while maintaining concordance with 123I-MIBG. These findings highlight 18F-MFBG PET as a powerful theranostic tool that could improve staging and treatment planning for children with neuroblastoma and potentially broaden theranostics applications in neuroendocrine cancers. Continue reading here
New Radiotheranostic Targets Identified for Diagnosis and Treatment of Endometrial Cancer
Researchers have identified HER2 and CD24 as highly promising radiotheranostic targets for endometrial cancer, offering new opportunities for both diagnosis and therapy. In preclinical studies, PET imaging with probes against these biomarkers showed strong tumor uptake and contrast, particularly with CD24-targeted agents, while HER2 also demonstrated meaningful potential. These findings could pave the way for precision theranostics in an understudied cancer type with limited treatment options, improving outcomes for patients with advanced disease. Continue reading here
Cellectar Biosciences and ITM Enter Supply Agreement for GMP-Grade Actinium-225
Cellectar Biosciences has partnered with ITM Isotope Technologies Munich to secure a reliable supply of Actinium-225 (Ac-225), a critical isotope for targeted alpha therapy. The agreement will support clinical development of Cellectar’s radiotheranostic pipeline, including CLR 121225, which has shown strong preclinical activity against solid tumors such as pancreatic cancer. With Ac-225 in short supply globally, this collaboration ensures access to high-quality isotope production and highlights the growing role of alpha-emitting radiopharmaceuticals in advancing theranostics. Continue reading here
ARTBIO Announces FDA Clearance of IND Application for Lead Alpha Radioligand Therapy AB001
ARTBIO has received FDA clearance to begin clinical trials of AB001, a Pb212-based PSMA-targeted radioligand therapy for metastatic castration resistant prostate cancer. Early Phase 0 data from Norway showed encouraging biodistribution, and the upcoming Phase 1 U.S. trial will test its potential to deliver higher tumor radiation doses without added toxicity. Backed by its AlphaDirect™ Pb212 generator platform and distributed manufacturing network, ARTBIO’s program represents an important step toward advancing alpha-emitting theranostics for prostate cancer. Continue reading here
New Targeted Radiation Therapy Shows Near-Complete Response in Rare Sarcoma Patients
A novel theranostic approach using 90Y-FAPI-46 radioligand therapy has produced near-complete responses in three patients with malignant solitary fibrous tumors, a rare and hard-to-treat sarcoma. By targeting fibroblast activation protein (FAP), which was highly expressed in patient tumors, the therapy led to tumor shrinkage, disease stabilization, and significant symptom relief without serious side effects. These early but striking results highlight the potential of FAP-targeted theranostics and warrant larger clinical trials to confirm safety and efficacy. Continue reading here